Empowering proteins to better treat diseases
Our diversified product pipeline includes treatments for hematological and endocrine disorders, cancer and infectious diseases. We intend to commercialize these products independently and through strategic alliances with corporate partners.
Neutropenia / ARS
Granulocyte Colony-Stimulating Factor (G-CSF) stimulates production…
Learn more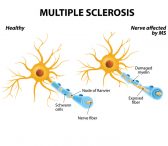
Multiple Sclerosis
Multiple Sclerosis is a chronic central…
Learn more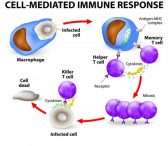
Cancer / Alzheimer’s Disease / ARS
White blood cells are critical for…
Learn more
Fungal Infections
Fungal infections can be life threatening,…
Learn more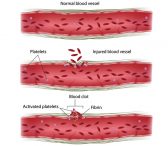
Bleeding Disorders / ARS
Platelets are blood cells responsible for…
Learn more
Anemia
Anemia (low numbers of red blood…
Learn more

Technology
Pipeline
BioDefense
News
Bolder BioTechnology Announces Positive Preclinical Data for Use of BBT-032 to Inhibit Growth of the SARS-CoV-2 Virus
Boulder, Colorado – August 25, 2020 - Bolder BioTechnology, Inc. announced today that preclinical studies have shown that its proprietary long-acting interferon beta analog, BBT-032, strongly inhibits growth of the SARS-CoV-2 virus…
read moreBolder BioTechnology Announces Scientific Presentations at the 2019 Radiation Research Society Annual Meeting
Boulder, Colorado – November 7, 2019 - Bolder BioTechnology is pleased to announce that three scientific presentations describing use of the company’s novel interleukin-11 analog BBT-059 to treat acute radiation syndrome were…
read moreBolder BioTechnology Receives NIH Grant to Advance Development of Novel Treatments for Acute Radiation Syndrome
Boulder, Colorado – July 18, 2019 - Bolder BioTechnology, Inc. announced today that it has been awarded a two year Small Business Innovation Research Grant totaling $593,507 from the National Institute of…
read moreBolder BioTechnology Announces FDA Orphan Drug Designation for BBT-059 for Acute Radiation Syndrome
Boulder, Colorado – June 12, 2019- Bolder BioTechnology, Inc. announced today that its long-acting IL-11 analog, BBT-059, has received Orphan Drug designation from the Food and Drug Administration for treatment of Acute…
read moreBolder BioTechnology to Present Preclinical Data for Three Research Programs at Acute Radiation Syndrome Meeting
Boulder, Colorado – October 24, 2018- Bolder BioTechnology, Inc. announced today that Dr. Joe Cox, Company President, will give an oral presentation at the “A Poly-Pharmacy Approach to Mitigate Acute Radiation Syndrome…
read moreBolder BioTechnology Announces Presentation at Radiation Injury Treatment Conference
Boulder, Colorado – September 6, 2018 – Bolder BioTechnology, Inc. announced today that Dr. Joe Cox, Company President, gave an oral presentation at the “Growth Factors and Other Cytokines for Treatment of…
read moreBolder BioTechnology Announces Positive Results from Phase 1 Clinical Trial of BBT-015, a Long-Acting G-CSF Analog, in Healthy Volunteers
Boulder, Colorado – April 27, 2018- Bolder BioTechnology, Inc. announced today that it has completed a Phase 1 clinical trial of BBT-015, a proprietary long-acting granulocyte colony-stimulating factor (G-CSF) analog, in healthy…
read moreBolder BioTechnology Announces Initiation of Phase 1 Clinical Trial of BBT-015 for Treating Neutropenia and Acute Radiation Syndrome
BOULDER, Colo., Aug. 2, 2017 /PRNewswire/ -- Bolder BioTechnology, Inc. announced today that it has commenced dosing of patients in a Phase 1 clinical trial of its proprietary long-acting granulocyte colony-stimulating factor (G-CSF) analog, BBT-015.…
read more
